Work-in-Process Tracking
CPCON delivers real-time WIP tracking solutions that provide complete visibility into production flow, enabling better scheduling, quality traceability, and continuous improvement.
Request a Demo
See our tracking solutions in action
Production Tracking Challenges
Manufacturing operations need real-time visibility to optimize flow, ensure quality, and meet delivery commitments. Our WIP tracking solutions address these critical needs.
Production Visibility Gaps
Without real-time tracking, managers cannot see where work orders are in the production process, making it impossible to provide accurate delivery estimates or identify bottlenecks.
Cycle Time Uncertainty
Manual tracking methods cannot capture accurate cycle times at each workstation, preventing identification of process inefficiencies and capacity constraints.
Quality Traceability Issues
When defects are discovered, tracing back to the specific batch, operator, or process step is difficult without automated tracking, complicating root cause analysis.
WIP Inventory Accumulation
Excess work-in-process inventory ties up capital and floor space. Without visibility, WIP builds up at bottleneck stations while other areas sit idle.
Schedule Adherence Problems
Production schedules become meaningless when actual progress cannot be tracked in real-time, leading to missed deadlines and expediting costs.
Manual Data Entry Burden
Operators spending time on paperwork and data entry reduces productive capacity and introduces errors that compromise production reporting accuracy.
Complete WIP Tracking Platform
Purpose-built hardware and software for tracking work orders through every stage of your production process.
Production Flow Tracking
Automated tracking of work orders through each production stage using RFID readers at workstations, providing real-time visibility into production progress.
Manufacturing Success Stories
See how manufacturers across industries have transformed their production operations with CPCON WIP tracking.
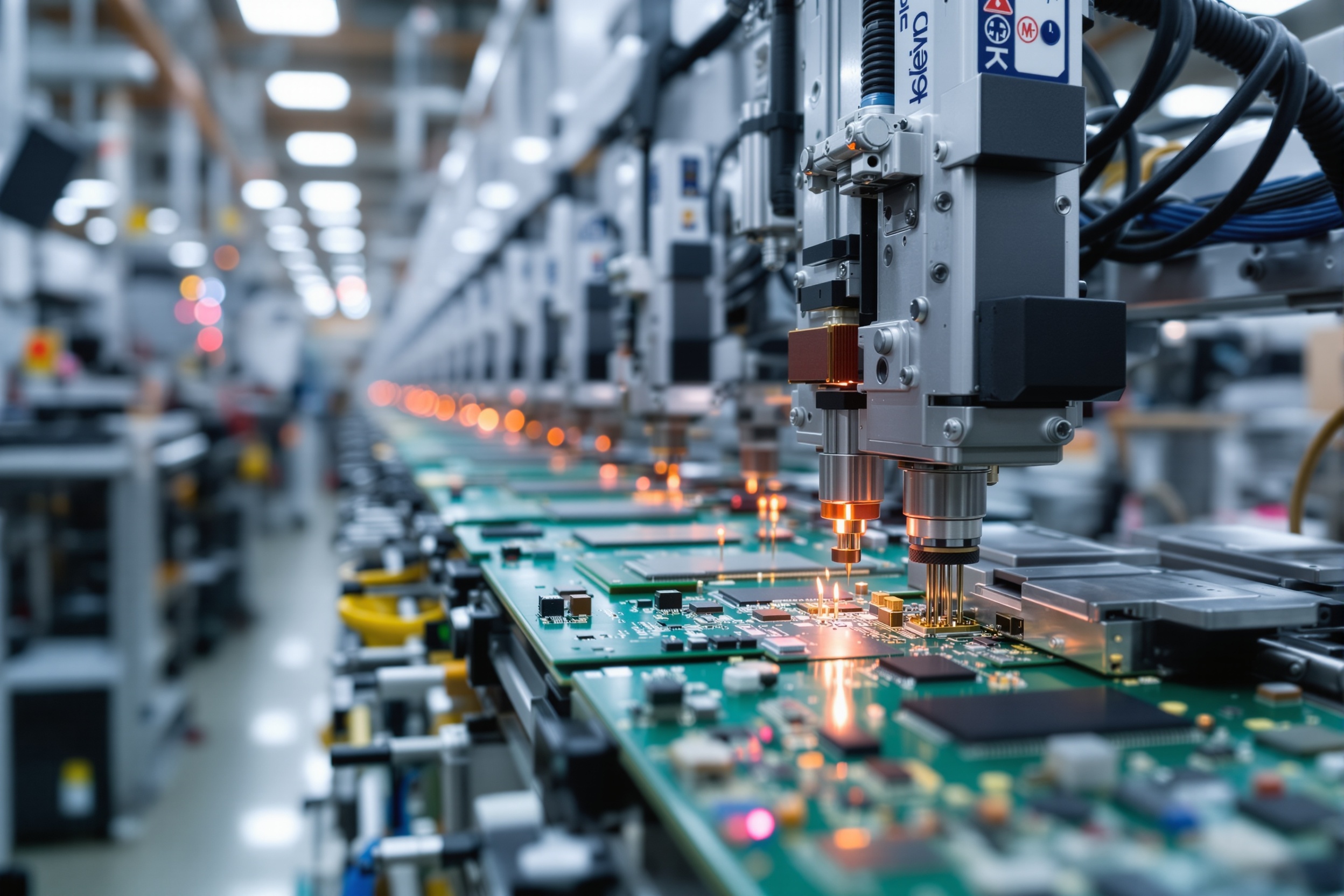
PCB Assembly Line Tracking
Implemented RFID-based WIP tracking across SMT and assembly lines, achieving real-time visibility and reducing cycle time variability.
Aircraft Component Manufacturing
Deployed WIP tracking for complex aerospace components with full traceability, meeting AS9100 requirements and improving on-time delivery.
FDA-Compliant Production Tracking
RFID-based device history record system for medical device manufacturing, ensuring FDA 21 CFR Part 820 compliance with automated documentation.
Latest Insights
Expert guidance on WIP tracking implementation and best practices
Real-Time WIP Tracking: A Lean Manufacturing Essential
How real-time work-in-process visibility eliminates waste, reduces cycle times, and drives continuous improvement.
RFID vs. Barcode for Production Tracking
A comprehensive comparison including performance metrics, cost analysis, and decision framework.
Traceability Requirements in Regulated Industries
Understanding and implementing traceability systems that meet regulatory requirements.
